Computerized System Validation (CSV)
Computerized System Validation (CSV)

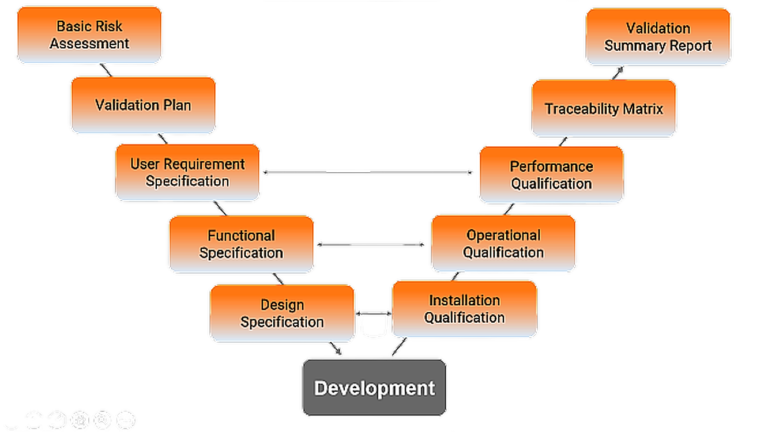
Computerized System Validation
In the ever growing life sciences landscape, the dependence on computer systems has increased, thereby requiring the validation of these systems. 1STEP has experts that work with our clients to validate the entire computer systems from a simple PLC based system to a complex enterprise level applications like ERP Applications
1STEP has the knowledge and experience to help the customers assess and define their compliance and validation requirements.
Our dedicated pharmaceutical and computer systems division are uniquely qualified to optimize any validation effort by applying quality validation services and the most current technology
- Comprehensive GAP & RISK Analysis
- Most appropriate & latest GAMP, 21 CFR Part 11, EU Annex 11 guideline based Compliance services for your new / legacy systems
- Validate Laboratory Equipment Software, IT systems, Network validation as per GAMP
- Validate Building Management & Environment Control Systems
- Validate DCS / SCADA / PLC Based Systems
- Database Systems including ERP such as SAP & LIMS
- Equipment validation
- Technical support during regulatory compliance audits

IT Infrastructure Qualification
- IT Infrastructure Planning
- IT Infrastructure Audits
- Data Center Audits
- Developing IT procedures and policies
- System Administration
- Security
- User Management
- Data and Application Backup and Restoration
- Document Management
- Configuration Management
- Release Management
- Preventive Maintenance Disaster Recovery and Business Continuity
Spreadsheet Validation
Spreadsheets are used extensively by several departments of Life Sciences companies to perform or support GxP regulated activities, thereby requiring these spreadsheets to be validated.
1STEP Works provides both documentation and execution support for spreadsheet validation.

